Did You Know…? A medieval sword weighed about the same as a bag of sugar.
Today we had some special visitors from DiscoverE at the University of Alberta. They were here to do some Chemistry demonstrations for us. First we discussed the differences between physical and chemical changes. So far we have only been working with physical changes. Physical changes can be reversed. That is why, using our separation techniques that we learned in class, we were always able to separate the materials into their original form. Using strategies like decanting, evaporating, filtering, etc. helped us to reverse physical changes. Another great way to think about a physical change is to think about the states of matter. Water changes form. It can cool (condense) from a gas to a liquid. It can freeze into a solid. It can then melt into a liquid again. It can even evaporate back into a gas. Each of these changes can be reversed. It is still just water, it is just in a different state, and using the appropriate strategy, we can bring it back to its original state. Chemical changes cannot be reversed. Today we saw three chemical changes, and we learned to look for signs that a chemical change had occurred. A change in temperature, a gas being produced, an odor being produced, a light being produced, a change in colour, and a precipitate forming are signs that a chemical change has occurred. We saw Keegan and Sarah (our presenters) create “Elephant’s Toothpaste,” a chemical reaction that demonstrated a colour and temperature change, as well as produced as a gas (in the form of bubbles). We saw them burn some powder in a combustion chamber, producing heat, light, an odor, and gas. Finally we created our own reaction. The first reaction we created was exothermic. The powder heated up when it was added to the water. We also saw the indicator (bromthymol blue) change colour (demonstrating a chemical reaction had occurred). The solution turned from a neutral to an acid. The second reaction we created was endothermic. The powder cooled down and bubbled when added to the water solution. The indicator (bromthymol blue) also changed once again, indicating that our solution had now turned from an acid to a base. Thanks for your great attentive listening today everyone. Want to attend a DiscoverE Summer Camp? Visit the link below for more information:
http://discovere.ualberta.ca/en/SummerCamps/RemoteandRuralCamps/FortMcMurrayCamps.aspx
Don’t forget that we have a Math test next week on Wednesday. Even though it is a long weekend. Students are all encouraged to study and prepare for their test. Students took home the following study guide today:
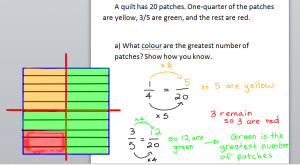
Fractions – Problem of the Day
Students were encouraged to bring home their Math Journals, which contains the answers for all the questions in the Study Guide. You can also use your Math textbook (remember, we do have access to an online version – see right) or your Mathletics to get some extra practice over the weekend. An example solution for one of the problems in the study guide is posted below with today’s pictures.
Agenda:
Read 20 minutes
Math:
- Math Help – Wednesdays from 3:00 – 4:00 pm
- Mathletics Meeting – Thursday, 6:00-7:00 pm
- Fractions and Decimals Test – Wednesday
Health:
- Pg. 77 (next Monday)
Google Classroom Form – ASAP
Grade 5/6 Floor Hockey – Monday (3:00-4:00 pm)
Yearbook Orders – May 29th ( ybpay.lifetouch.com and enter Yearbook ID code: 10359315)
Busing Intent Forms – due ASAP
Mega Boyz – May 27th (Permission Forms ASAP)
Always Changing Unit – May 27th, girls (Permission Forms ASAP)
* More information on this unit can be found at: http://www.phecanada.ca/programs/always-changing/vibrant-faces/always-changing
Spring Pictures – due May 19th




hey mrs.b, are u going to be on? it ok if u cant
mathletics
I’m late, but I am on! Sorry everyone!
Thanks for being on!
I find it weird we are the only ones on normaly (not all the time!)
That’s okay. That just means you are getting lots of extra math practice. Live math is a great way to increase your math fluency.
thanks for playing mathletics with me Mrs.B! see you Monday 😀
Have a great long weekend!
you too 🙂
Hi sorry I couldn’t come because everyday at 5:30 or 6:30 I go and play soccer I Alec in Gr5 and I came home at 8:45.
That’s okay! We’ll schedule a new one next week, and then maybe we’ll see you then 🙂
Referring the question, “Identify the equivalent fractions from 3/4, 8/12, 6/8, 6/9, 9/12”, we discussed answer as 3/4 = 6/8 = 9/12. Does this have one more answer as 8/12 = 6/9?
Good eyes! When we marked this problem in class, I used 3/4 and found equivalent fractions that could be reduced to 3/4 (6/8, 9/12). However, you are correct, 8/12 = 6/9! Both of these fractions could be reduced to 2/3! Great job! It is great to see that you are thinking so deeply about these problems!
Actually it was my father who said to ask this question.
My dad told me something about GCD {Greatest common divisor} and LCD {Lowest common denominator} do we have this concept for this year or next year.
No, you do not need to know this concept this year. However, if you understand it already, feel free to use it to help you with the test on Wednesday. An understanding of the GCD and LCD will help you to figure out equivalent fractions.